How Microfluidics Is Transforming Enzyme Engineering
Blog
November 11, 2025
1min
.webp)
How Microfluidics Is Transforming Enzyme Engineering
Understanding the Role of Microfluidics in Modern Biocatalysis
Over the past few decades, advances in biotechnology have made it possible to modify enzymes for use in industrial applications. Enzymes are biological catalysts that drive chemical reactions with high specificity and efficiency. They play essential roles in producing food ingredients, pharmaceuticals, and specialty chemicals.
Despite these advantages, the process of discovering and improving enzymes is often slow. A single enzyme may need to be tested in millions of variations to identify one with the right activity or stability for a specific process. Traditional screening methods rely on robotic platforms or multi-well plates that can handle thousands of reactions per day. While this is effective, it limits how much biological diversity can be explored.
Microfluidics provides a new way to perform enzyme discovery and engineering. By allowing researchers to test millions of enzyme candidates in miniature reaction systems, it significantly increases the screening throughput that can lead to higher chance of success in each round.
What Is Microfluidics
Microfluidics is the study and application of fluids in channels smaller than a millimetre in diameter. These channels are etched or moulded into materials such as glass, polymers, or silicon to form miniature laboratory systems. A microfluidic device can control and move fluids in precise patterns, enabling thousands or even millions of chemical reactions to occur in parallel.
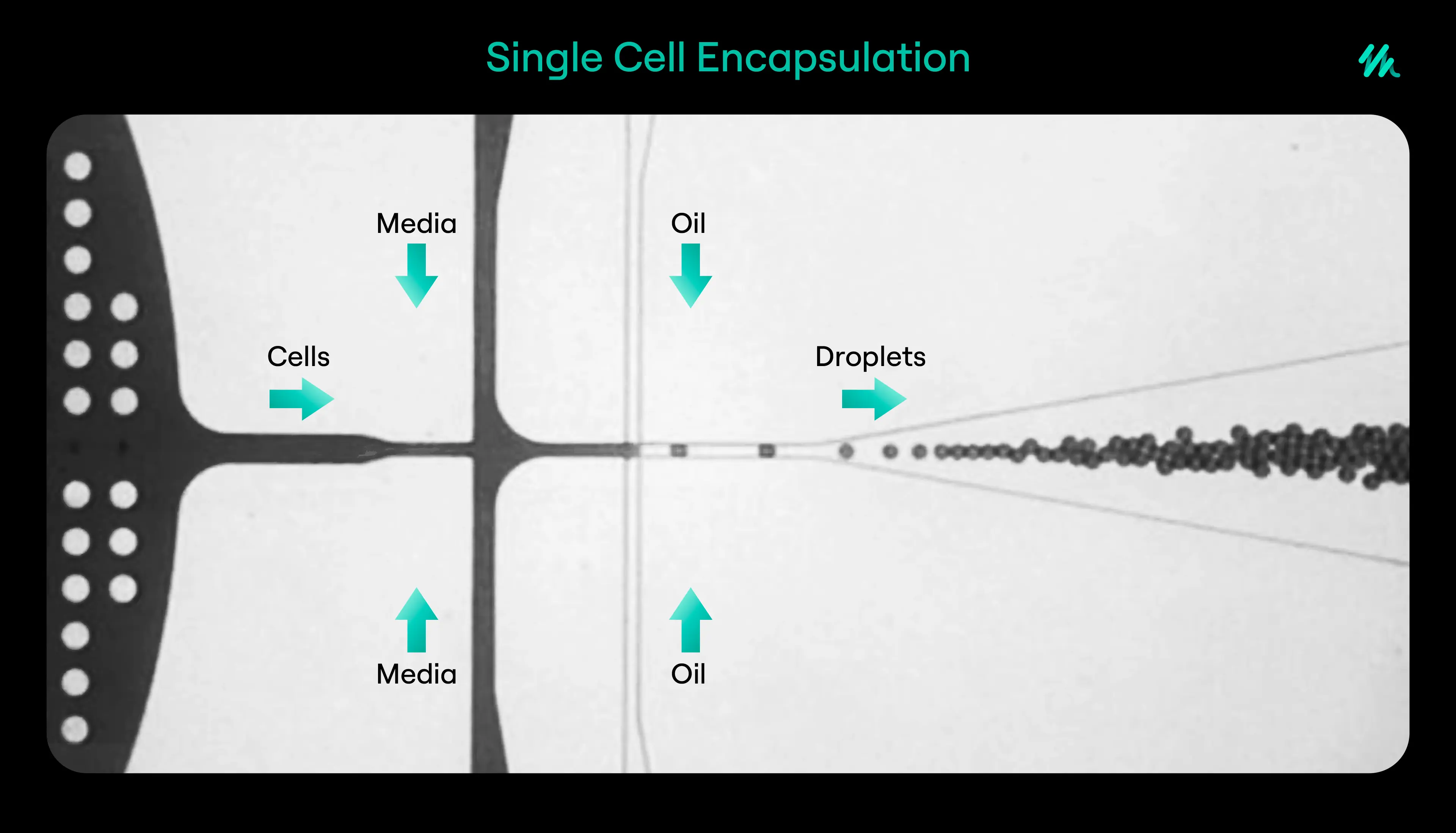
In biotechnology, microfluidics is often used in the form of droplet-based systems. In these systems, tiny droplets of water are generated within an oil medium. Each droplet acts as a microreactor that can contain an individual enzyme variant, its genetic material, and the chemical substrate it acts upon.
This method allows experiments to be performed in extremely small volumes, often in the range of picolitres. The droplets are uniform in size and can be produced at rates of thousands per second. Because each droplet is separated from its neighbours, the reaction taking place in one does not interfere with others.
This separation is important for enzyme engineering because it maintains a clear link between the genetic code of an enzyme and the results of the reaction it catalyses. In practical terms, it means that researchers can identify which DNA sequence produces the best-performing enzyme.
How Microfluidics Accelerates Enzyme Discovery
Conventional enzyme screening methods involve manual or robotic transfer of liquids into microtiter plates. Each well acts as a reaction container, and enzyme performance is measured by various analytical tools. Although effective, this approach is limited by the number of wells that can be processed and by the large quantities of reagents and consumables required for each test.
Microfluidic systems overcome these constraints by shrinking reaction volumes and running thousands of experiments simultaneously. Each droplet in a microfluidic device can serve as a miniaturized laboratory, allowing millions of tests to be conducted in a single day.
A typical workflow begins with the generation of a large library or set of libraries of enzyme variants. The genetic material encoding these enzymes is transformed into microbial hosts such as bacteria or yeast which then are fed into a microfluidic chip. As the solution flows through the device, individual cells are encapsulated into droplets together with the chemical reaction reagents.
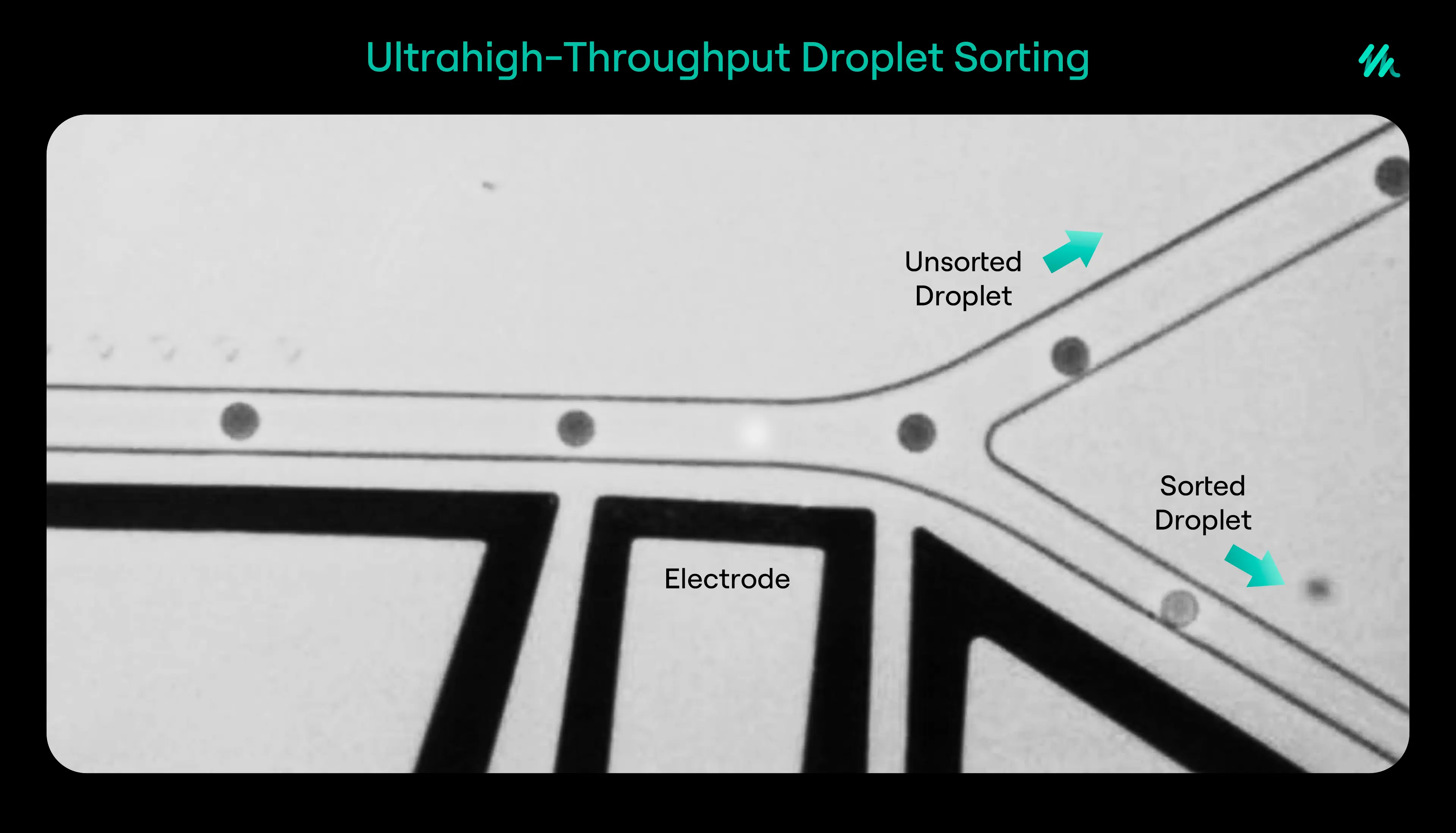
When an enzyme within a droplet catalyses a reaction, the resulting product emits a measurable signal, such as fluorescence. Detectors built into the system record the signal strength, and droplets containing high-performing enzymes can be sorted and collected for further study.
This process enables rapid and high-precision screening. It also minimizes the amount of reagents and consumables used, making the approach more sustainable and cost-effective. In addition, the fine control over reaction conditions allows scientists to test how enzymes behave under different temperatures, pH levels, or solvent environments, which are important parameters for industrial applications.
Advantages of Microfluidic Systems
Microfluidic platforms provide several key advantages for enzyme discovery and development.
High throughput. Microfluidic systems can process thousands of droplets per second, greatly increasing the number of enzyme variants that can be tested. This allows researchers to explore a wider range of possible mutations and identify improved enzymes more efficiently.
Reduced reagent use. Each droplet has a volume that is a million times smaller than a standard laboratory well. This reduces the amount of reagents and samples needed for experiments, lowering costs and waste generation.
Precision and reproducibility. The design of microfluidic devices allows precise control over droplet size, reaction time & conditions, and mixing. Experiments can be reproduced under highly consistent conditions, improving the reliability of screening results.
Automation. Many stages of enzyme screening — such as droplet generation, incubation, detection, and sorting — can be integrated into a single automated system. This reduces manual handling and human error while improving speed.
Compatibility with cell-free systems. Microfluidics is also suitable for cell-free enzyme expression. In this setup, the enzyme is synthesized directly from DNA templates inside the droplet without requiring living cells. This method shortens the time between design and testing and can be used for enzymes that are toxic or unstable in cellular systems.
Applications in Industry
The ability to evaluate enzymes at high speed and low cost has made microfluidics a valuable tool for industrial biotechnology.
In biocatalysis, microfluidic systems are used to identify enzymes that can perform complex chemical reactions more efficiently than traditional catalysts. These reactions include oxidation, reduction, hydroxylation, and transamination, which are central to the synthesis of pharmaceuticals and fine chemicals.
In the food and nutrition sector, microfluidics-based enzyme screening has led to the development of enzymes that can convert plant-derived compounds into functional ingredients. These include natural sweeteners, flavour enhancers, and nutritional supplements that are produced using environmentally friendly processes.
In sustainable manufacturing, microfluidics enables the design of enzymes that operate under mild reaction conditions. This allows industries to reduce their reliance on heavy metals, solvents, and high-energy input, leading to cleaner production methods and lower carbon footprints.
Microfluidic technologies are also expanding into fields such as polymer degradation, and biosensor development. Their precision and scalability make them ideal for integrating biological reactions into modern production pipelines.
Challenges in Implementation
Although microfluidics offers many advantages, several challenges remain before it can be routinely integrated into R&D workflows for enzyme discovery and optimization.
The design and fabrication of microfluidic devices require specialized expertise and equipment. The materials used in chip production must be chemically compatible with biological samples and capable of maintaining stable droplet formation. Furthermore, droplet-based systems can be sensitive to variations in pressure, temperature, and surface tension, which must be tightly controlled for consistent operation.
Data management is another consideration. Microfluidic systems can produce vast amounts of experimental data in a single run. Efficient data analysis tools, often powered by machine learning algorithms, are essential to interpret these results and guide subsequent rounds of enzyme optimization.
Efforts are underway to standardize microfluidic device fabrication and operation. Advances in 3D printing and modular chip design have made it easier to produce customizable systems. In parallel, improvements in optical and electrical detection methods are increasing throughput and sensitivity. These developments will continue to make microfluidics more accessible to laboratories and production facilities around the world.
The Future of Enzyme Engineering
Microfluidics has fundamentally reshaped how scientists discover and improve enzymes. By miniaturizing experiments, enhancing throughput, and providing fine control over conditions, it allows researchers to explore the vast landscape of protein possibilities more efficiently than ever before.
At Allozymes, this is at the core of how we operate. Our proprietary microfluidics platform combines ultrahigh-throughput screening with data-driven enzyme design to accelerate discovery and development from concept to scale. By integrating microfluidics, AI, and enzyme engineering, we identify, evolve, and optimize biocatalysts for industries ranging from pharmaceuticals to nutrition, cosmetics, and specialty chemicals.
As the technology matures, these integrated approaches will continue to close the gap between laboratory discovery and industrial manufacturing. Together, they enable cleaner, smarter, and more sustainable biosolutions that redefine what enzymes can achieve in the real world.
For updates, insights, and new releases, subscribe to our quarterly newsletter and follow Allozymes on LinkedIn.